.svg)
MectaCem-X is the Medacta-branded bone cement supplied by Tecres Spa, a leading company in the manufacturing of acrylic resins. MectaCem-X is a safe and reliable bone cement with excellent mechanical performance and more than 30 years of clinical heritage [1,4]. With MectaCem-X, Medacta provides a modern bone cement that is easy to handle in modern vacuum mixing systems.
Thanks to an exclusive process manufactured by Tecres, MectaCem-X powder particles are consistent in shape and size, in contrast to other commercially available cements (see particle comparison). These characteristics result in uniform spheres with no irregularities that yield decreased total surface area.
This feature means less liquid monomer is needed to prepare the cement dough. Since the monomer is the most dangerous component, the revolutionary 3:1 powder/liquid ratio puts MectaCem-X in a different league from the traditional 2:1 ratio, providing significant and proven advantages to the surgeon, the O.R. staff, and the patients.
MectaCem-X has proven to be less toxic than its competitors with higher amount of monomer. The monomer release into patients is reduced by 30%, as well as the exposure of surgeon and O.R. staff to toxic fumes[5].
MectaCem-X has a 10% reduction in the polymerization temperature compared to other commercially available formulations. This results in a lower maximum curing temperature, thus reducing thermal damage to surrounding bone and soft tissues[6].
Bone cement shrinkage depends mainly on the amount of monomer present in the cement mixture and contributes to the loosening of the prostheses[7]. MectaCem-X shrinks well over 50% less than its competitors with 2:1 powder/liquid ratio, thus greatly improving implant fixation[8].
The MectaCem-X offering comprises an extensive range of high-quality bone cements that allow for great flexibility with respect to different surgeon’s needs.



MectaCem-X with Gentamicin is pre-blended with 1.0 g (2.5%) of gentamicin base, which has a broad antibacterial spectrum and long-lasting antibacterial protection. Antibiotic loaded bone cements are proven to reduce the risk of infection to 1.2% compared to 2.3% when no antibiotic is used in the bone cement[9].
MectaCem-X with Gentamicin has a fast and high release of antibiotic extending over several days. Antibiotic elution is particularly strong during the first few hours post-operatively, the time when the risk of infection is at its greatest[6,10].
Porosity has been found to be the major cause of decreased mechanical strength and fatigue life of bone cement[11]. The high consistency in shape and size of MectaCem-X powder particles allows for obtention of a more dense structure that traps less air, resulting in a bone cement with a very low porosity.
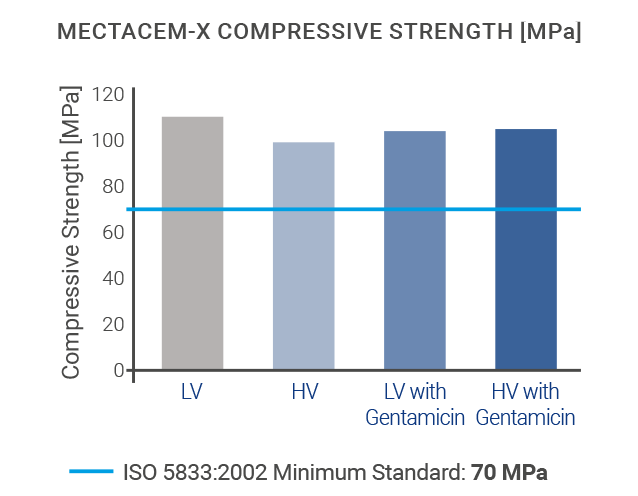
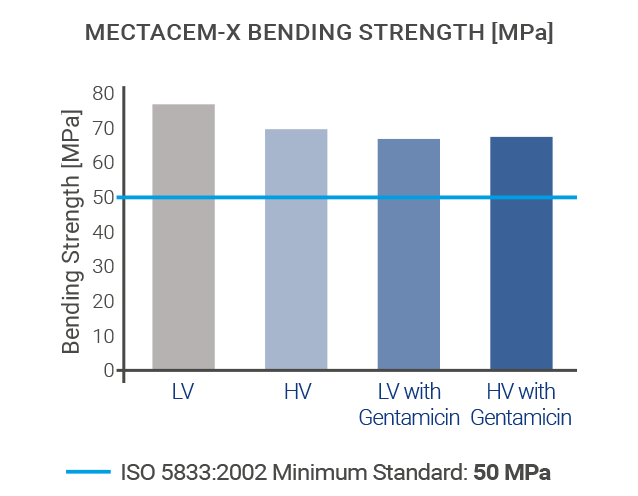
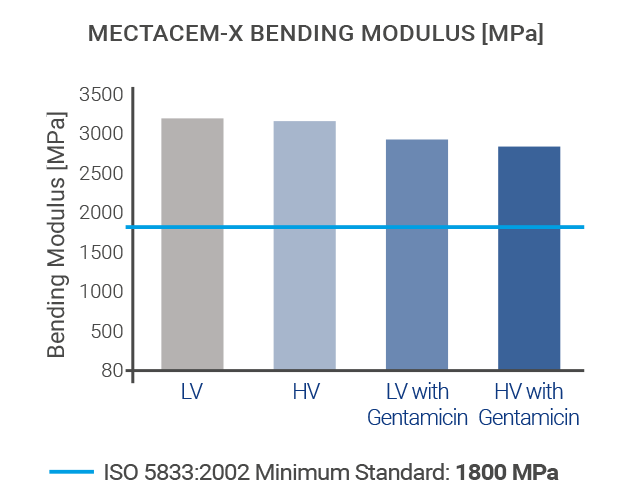
The mechanical properties of MectaCem-X have successfully been tested for compressive strength, bending strength and bending modulus according to international standards[6,12].
Bone cement setting time is affected by several factors, including storage and O.R. temperatures, humidity, mixing conditions, and mixing speed[13]. All these conditions can be affected by variables in the operating room environment, which could potentially result in an unpredictable working phase and overall setting time. Controlling the variables can limit complications and may lead to more reproducible results. The times here displayed refer to a manual mixing. Higher temperatures will result in a shorter manual application phase and faster setting time[6].

To facilitate orthopaedic bone cement handling, Medacta has added to its product portfolio a comprehensive range of options for cement and bone preparation.
[1] Bialoblocka-Juszczyk E, Cristofolini L, Erani P, Viceconti M., Effect of long-term physiological activity on the long-term stem stability of cemented hip arthroplasty: in vitro comparison of three commercial bone cements. Proc Inst Mech Eng H. 2010;224(1):53-65. October 2010.
[2] Rossi R, Bruzzone M, Bonasia DE, Ferro A, Castoldi F., No early tibial tray loosening after surface cementing technique in mobile-bearing TKA. Knee Surg Sports
Traumatol Arthrosc. 2010 Oct;18(10):1360-5. Epub 2010 Jun 10.
[3] Li ZJ, Zhang K, Yang H, Liu Y, Lü JQ., Intraoperative monitoring for safety of total hip arthroplasty using third-generation cementing, January 2009.
[4] Söderlund P, et al. 10-year results of a new low-monomer cement: follow-up of a randomized RSA study. Acta Orthop. 2012 Dec;83(6):604-8. doi: 10.3109/17453674.2012.742392. Epub 2012 Nov 1.
[5] Neotron Laboratory, Gatti G, Modena, Italy. 1989.
[6] Data on File at Tecres S.p.a..
[7] Orr JF, Dunne NJ, Quinn JC. Shrinkage stresses in bone cement. Biomaterials. 2003 Aug;24(17):2933-40. doi: 10.1016/s0142-9612(03)00055-3. PMID: 12742733.
[8] Trieu H, et al. Comparative measurement of shrinkage of 5 commercial cements prepared under vacuum mixing. In: Grasse F, et al [ed]. Bone cements in the year 2000: state of the art and prospects. Italy: Varese; 2000. 23.
[9] Parvizi J, Saleh KJ, Ragland PS, Pour AE, Mont MA. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthopaedica, 2008; 79 (3): 335-341.
[10] Macdonald DA. The infected joint replacement: Prevention, diagnosis and treatment. Curr Orthop 1995; 9: 21-27.
[11] Wang JS. (2005) The Benefit of Vacuum Mixing. In: The Well-Cemented Total Hip Arthroplasty. Springer, Berlin, Heidelberg.
[12] ISO 5833, Implants for Surgery - Acrylic Resin Cements (2002).
[13] BAC-DATA, The Bacteriological Report, Vol. 1 (Dec 1986-Feb 1987).